
Professional master’s degree for students seeking a unique specialization in an entrepreneurial, innovative field.
The students of this program are trained on the processes involved in life cycle medical device development to enable global product launches.
Who Should Apply
Ideal candidates for this program include:
- Aspiring students who wish to pursue a career in Biomedical Product Development such as medical devices.
- Early-career professionals working in biomedicine.
- High-performing recent graduates from undergraduate disciplines such as:
- Engineering, Science, Business & Medicine.
Requirements
- Classroom and design studio courses will be taught on the Georgia Tech campus in Atlanta.
- Clinical/experiential courses may be conducted at allied medical institutions and/or selected biomedical industry sites throughout the greater Atlanta area.
Schedule
The MBID master’s program is a one-year residential program that is completed in three sequential semesters over 12 months. Candidates enroll in the fall semester and take four courses (fall & spring semester) and three courses (summer semester).
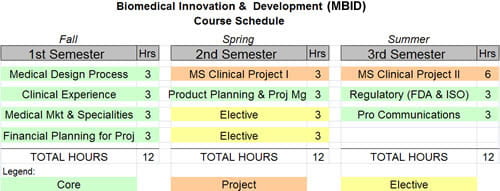
Course of Study
The following curriculum presents the 12-month course of study required to earn the MBID professional master’s degree. Learn More
Tuition & Fees
Please visit the Office of the Bursar for the current tuition rate and fees. Learn More
FAQs
Some of the more common general questions about the MBID master’s program. Learn More
Program Brochure
Brochure is available for download in Adobe Acrobat Format. Learn More
Unique Highlights of the Program
The students of this program are trained on the processes involved in life cycle medical device
development to enable global product launches. The training comprises the following functions and domains of expertise.
Pre-Clinical R&D
Comprising the horizon from early concept evaluations and concept prototyping through
pre-clinical development and testing for regulatory
submission.
Regulatory and Clinicals
Covering all aspects of
preparing submissions for global approvals such as 510ks, IDE/PMAs, CE file and country-specific
submissions as well as conducting clinical studies and preparing reports for regulatory submissions.
Quality Assurance
Covering the elements of design controls, change controls, non-conformances, CAPA, etc.
Manufacuturing Scale-Up and Validations
For commercial release in global markets.
ACCESSIBILITY INFORMATION
Per accessibility compliance standards, this page may have links to files that would require the downloading of additional software:


